Analytical Methods 101 – SEM
We use a variety of different analysis methods in our project, which we would like to introduce to you in this format. After presenting the XRF analysis method in the last article of this series, we would now like to introduce you to the scanning electron microscope (SEM). SEM is a powerful tool used in scientific research and industry to measure the chemistry of points of interest, examine the surface of materials at high magnification or to create element distribution maps of a sample. It works by using a beam of electrons to scan the surface of a sample, producing detailed images that reveal the structure and composition of the material.
Similar to XRF, the elemental analysis of the SEM also works with energy dispersive X-ray spectra, also known as EDX. In brief: A mineral grain of interest is bombarded with electrons; an electron close to the core is knocked out of its position; the gap is filled by an electron from a higher orbital and must release energy when changing position, this energy is element-specific. This allows researchers to identify the elements present in the sample and determine their relative concentrations.
We use this method in environmental and waste mineralogy. For example, it is used to analyse the distribution of pollutants in mineral waste or to test individual mineral grains for their pollutant content. With the SEM results, we can also determine minerals, and subsequently estimate whether pollutants are firmly bound and therefore immobile or less firmly bound and therefore mobile. This important finding supports us, in the search for new alternative applications of the analysed material.
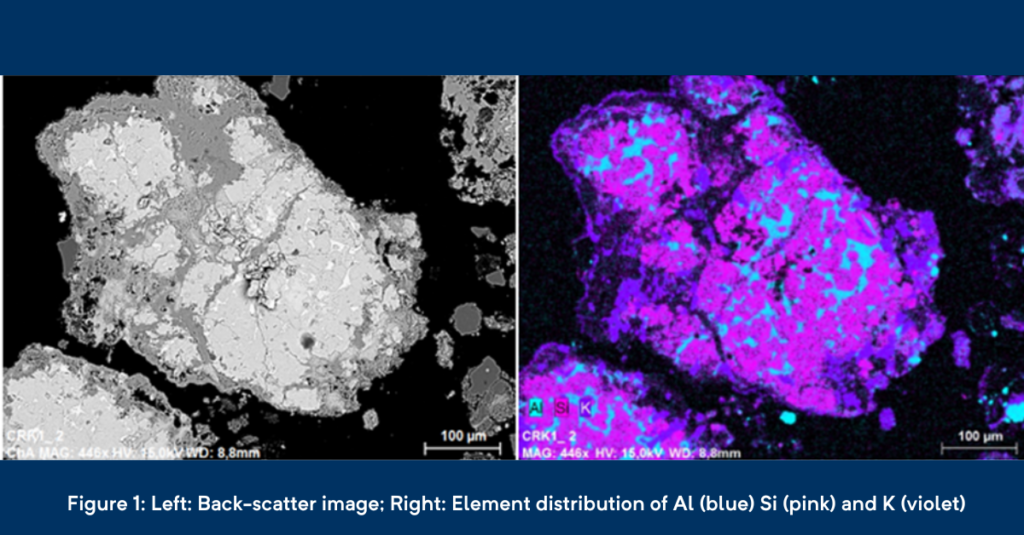
However, since pollutants do not play a role in spent refractory materials, we have focused on those elements that can directly hinder the further recycling of spent refractories. With SEM, we have created so-called element distribution maps (Figure 1, right). By evaluating these maps, we can make statements about the distribution of elements that might hinder the further recycling process like potassium (K). Other than that, we can also have a look on the distribution of recycling mineral phases and determine their size, their chemical content as well as their intergrowth structures. This helps us to optimise the recycling process.

Author´s Portrait
Florian Feucht
DI Florian Feucht is research associate at the Chair of Waste Management and Waste Treatment at the Montanuniversität Leoben and part of the Workgroup: “Environmental remediation and mineral waste”. Since 2023, he has been enrolled in the university’s PhD Program. He earned his master’s degree in Applied Geoscience from Montanuniversität Leoben, focusing on the chemical-mineralogical characterization of ladle slag. He completed his bachelor’s degree in Earth Sciences at the University of Vienna, with a thesis on the petrological study of mafic and ultramafic rocks. His research interests include the chemical mineralogical characterization of mineral wastes, mineralogy, slag mineralogy, recycling, and waste management.
Partner

