Analytical Methods 101 – XRF
In our blog, we don’t only give you updates on our research but also give you a bit of an insight into our specialist disciplines.
This posting will be of interest to you if you are interested in analytical methods, because today we will have a brief look at X-ray fluorescence analysis. This method is usually used to determine the total chemistry of a sample and has the great advantage of being easily reproducible and correct.
X-ray fluorescence (XRF) is a non-destructive, quantitative, as well as qualitative analysis, which reflects the concentration of the elements of the sample, often display in weight percentage (wt%) and in the form of oxides. XRF can measure all the elements whose atomic number is greater than or equal to four, i.e. boron. The detection limit, the smallest measurable amount of an element, of this method ranges for 1 – 10 ppm, whereas ppm stands form “parts per million” and can also be expressed as 0,0001 % as a more understandable unit.
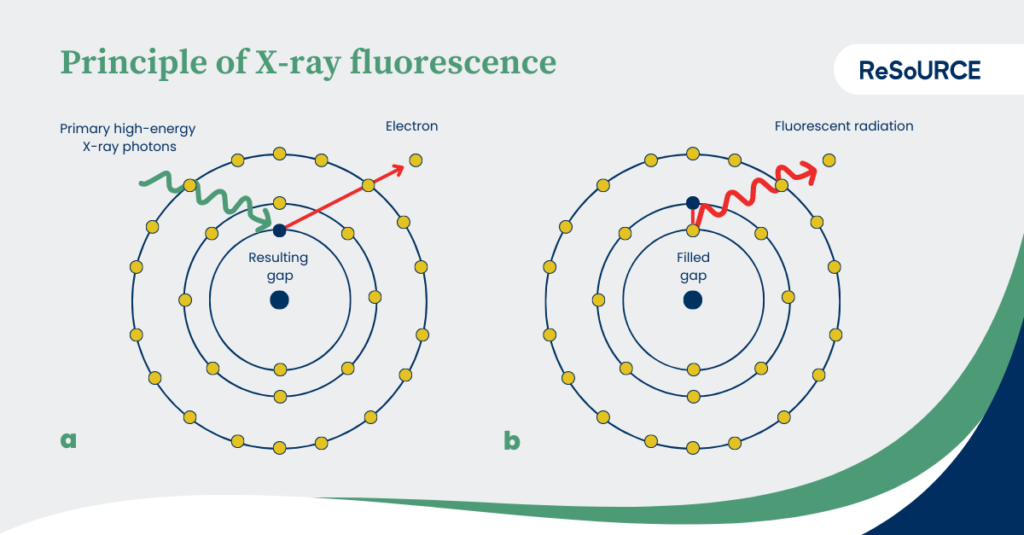
During the measurement, the atoms of a sample are bombarded with high-energy X-ray photons. If a photon hits an electron, it is shot out of its shell. The resulting gap is filled by electrons from energetically higher shells. By switching to an energetically lower level, energy in the form of fluorescent radiation, i.e. element-characteristic X-rays, is released and can be measured. This effect is also known as fluorescence.
To obtain analytical results, the sample must be homogenized in advance. This is done by crushing and grinding, whereby a particle size of < 100 µm is aimed for and in the best case is around 50 µm. After processing the sample material is pressed into pellets or fused and casted into glass disks.
Results are often displayed in form of a spreadsheet like you can see it in the picture below.
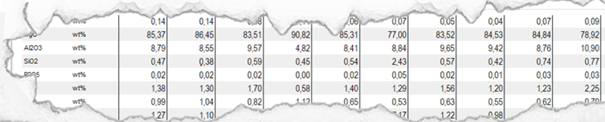
Figure 1 Preliminary XRF results of CRK 1
The here presented knowledge can be backed up by the following textbooks:
- German: Amthauer, G. & Pavicevic, M. K. (2000). Mikroskopische, analytische und massenspektrometrische Methoden. Physikalisch-chemische Untersuchungsmethoden in den Geowissenschaften: Bd. 1. Schweizerbart. S. 115
- English: Putnis, A. (1992). Introduction to mineral sciences. Cambridge University Press. P. 109
Featured image: Principle of X-Ray Fluorescence. Translated after Matthias Ahlfeld www.weltderphysik.de. (CC BY-NC-ND 3.0) Original: https://www.weltderphysik.de/gebiet/teilchen/licht/synchrotronstrahlung/roentgenfluoreszenzanalyse/roentgenfluoreszenzanalyse/ [accessed: 20230522]
Author’s Portrait
Florian Feucht
DI Florian Feucht is research associate at the Chair of Waste Management and Waste Treatment at the Montanuniversität Leoben and part of the Workgroup: “Environmental remediation and mineral waste”. Since 2023, he has been enrolled in the university’s PhD Program. He earned his master’s degree in Applied Geoscience from Montanuniversität Leoben, focusing on the chemical-mineralogical characterization of ladle slag. He completed his bachelor’s degree in Earth Sciences at the University of Vienna, with a thesis on the petrological study of mafic and ultramafic rocks. His research interests include the chemical mineralogical characterization of mineral wastes, mineralogy, slag mineralogy, recycling, and waste management.
Partner

